
Study Overview.
Improving vascular health to delay the onset of Alzheimer’s disease and related dementias is thought
to be a critical potential prevention strategy. However, because the links between cardiovascular
disease and Alzheimer’s disease pathology remain unclear, there are few specific vascular targets
for prevention.
MESA-MIND is focused on understanding how subclinical vascular disease may increase
the risk for dementia by creating vascular pathology in brain. This is especially important
for racial/ethnic minority groups in the United States who have a higher risk of developing both
cardiovascular disease and Alzheimer’s disease.
MESA-MIND will collect detailed cognitive testing
and brain imaging from MESA participants and will leverage the detailed vascular phenotypes collected
by MESA to determine how subclinical vascular disease may contributes to brain function
and the risk for Alzheimer’s disease later in life.
MESA-MIND Visit Components
Visit components may be completed in one day or scheduled over 2 days.
Blood Pressure: Resting blood pressure will be measured in the right arm after five minutes in the seated position using automated Dinamap.
Anthropometry: Height, weight, waist girth, and hip girth.
Blood Collection: 26 mL of blood are collected.
Arterial Stiffness/Distensibility: Performed using a brachial and leg cuff-based approach with Fukuda VaSera devices.
* Blood pressure, anthropometry, arterial stiffness, and phlebotomy will be measured in fasting state.
Questionnaires: Demographics, socioeconomic and psychosocial status, physical function, medical and
family history of dementia, medication use, alcohol intake, and smoking status are collected.
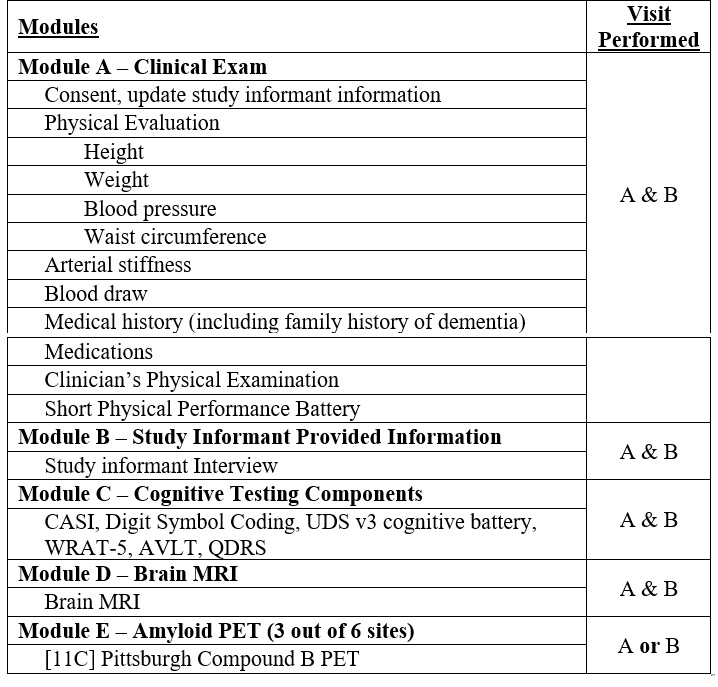
Cognitive Function Testing and Study Informant: Cognitive testing includes the National Alzheimer’s
Coordinating Center Uniform Data Set version 3 (UDS v3) cognitive battery, in addition to WRAT-5
(Wide Range Achievement Test – 5), AVLT (Auditory-Verbal Learning Test), and QDRS (Quick Dementia Rating System).
The participant also identifies a Study Informant who knows them well and can answer questions about their daily life.
The Study Informant will complete the QDRS, NPI-Q (Neuropsychiatric Inventory Questionnaire), and
FAQ/FAS (Functional Activities Questionnaire). The results of these measures in addition to other
data will be used by an adjudication committee to classify cognitive function.
Short Physical Performance Battery (SPPB): The extended version of the SPPB combines the results of
gait speed, chair stand and balance tests in order to assess the lower extremity functioning.
Brain MRI: Brain MRI protocol continues to protocol of the MESA Atrial Fibrillation Study (2017-2019)
and includes: 3D T1, 3D FLAIR, 3D T2, 3D ASL, DTI 30 dir, BOLD Rest, BOLD Breath Hold,
Breath hold compliant, QSM/SWI, and Dixon-T1 Vibe of tongue.
MESA-MIND Cognitive Battery
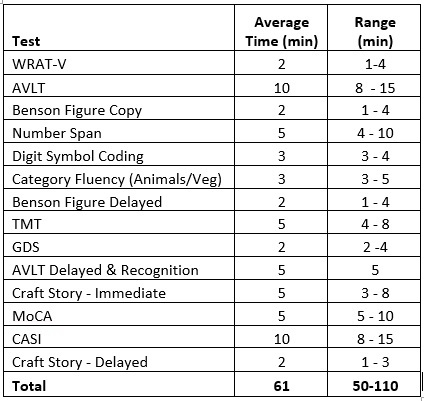
Telephone- and Video-administered Cognitive Batteries
MESA-MIND has adapted to continue recruiting during the global COVID-19 pandemic.
Since June 2020, MESA-MIND has offered participants the ability to complete a modified
version of the cognitive battery in a telephone interview (TCog).
In April 2021, MESA-MIND began offering another alternative, the video cognitive battery (VCog).
Tablets equipped with cellular service are shipped directly to participants’ homes. The tablets do
not require a WiFi connection or any setup by the participant, they arrive ready to use at the touch of a button.
After completing the TCog or VCog, participants are invited to complete MRI/PET imaging and
the in-person Module A components when it is safe to do so according to institutional COVID-19 safety guidelines.
MESA-MIND will continue to offer the TCog and VCog in Visit B
to optimize recruitment and participant safety.
MESA-MIND Ancillary Study Timeline
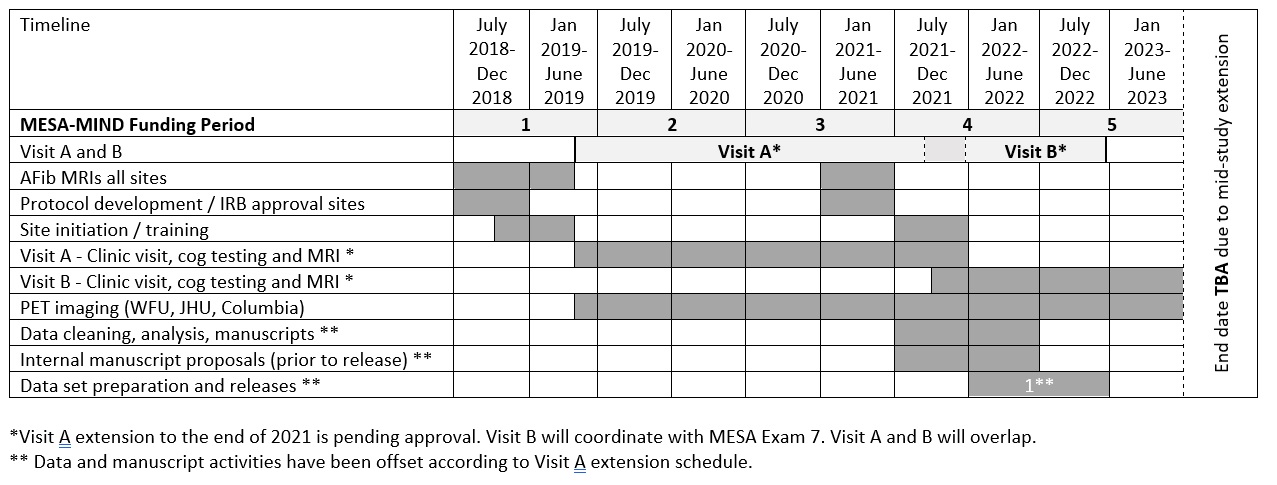
Site Specific Enrollment Targets for MESA-MIND
